At RegConsult, we understand the intricacies and demands of eCTD submissions in the pharmaceutical and healthcare industries. Our expert team is dedicated to ensuring your regulatory submissions are accurate, compliant, and delivered on time via our eCTD publishing services

Why choose Regconsult for your eCTD publishing needs?
Expertise: Our team comprises regulatory specialists with extensive experience in eCTD submissions. We stay updated with the latest regulations and guidelines to provide you with the most reliable service.
Customised Solutions: We recognise that every project is unique. That’s why we offer tailored solutions to meet your specific requirements, whether it’s a single submission or ongoing regulatory support.
Efficiency: Time is critical in regulatory submissions. With Regconsult, you can expect efficient processes and quick turnaround times, ensuring your submissions are on schedule.
Quality Assurance: We maintain rigorous quality assurance processes to guarantee the accuracy and completeness of your submissions, minimising the risk of errors or delays.
Client-Centric Approach: Your satisfaction is our priority. We work closely with you throughout the entire process, providing clear communication and proactive support to address any concerns or queries you may have.
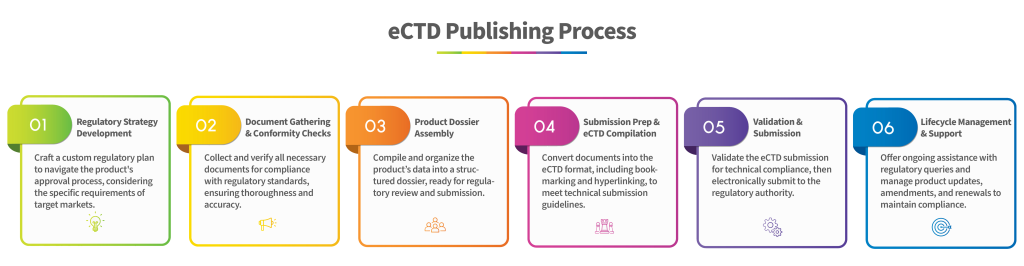
Partner with RegConsult for seamless eCTD publishing solutions that streamline your regulatory processes and drive your success in the pharmaceutical and biotech industries.
Contact us today to discuss your eCTD publishing needs and discover how we can support your regulatory goals.